Glue and stacks: The secrets of DNA ring polymers
Polymers are everywhere, not just in nearly all artificial materials surrounding us, such as plastic, paper or glass, but also in important components of the natural world, for example in wool and cotton. But that’s not all: Many proteins in the human body are polymers, even the DNA in every single cell is a polymer.
Both artificial and natural polymers can have very different shapes: linear, knotted, branched or ring-shaped. While linear polymers, for example cellulose or PVC, are very well researched, we know less about ring polymers. In nature, some types of DNA molecules are ring-shaped, such as plasmids (see info box) or chromosomes of certain bacteria.
The circular polymers have different properties. As they are rings, they also have several limitations. For example, two unlinked polymer rings must always remain unlinked. "Although these constraints are very difficult to incorporate in the so called 'polymer field theories' (see Info box below), they are also extremely exciting, as these characteristics affect the material properties", says Jan Smrek from the Computational and Soft Matter Physics Group at the University of Vienna.
Macromolecules derived from nature and designed in the laboratory
- Polymers are large molecules, so-called macromolecules, which are produced through the polymerization of small units, known as monomers. A polymer can be organized as a linear polymer or a branched one, depending on its structure.
- Natural polymers are materials that exist in nature or are derived from plants or animals, such as human proteins and nucleic acids, as well as cellulose, natural rubber, silk, or wool.
- Artificial polymers are derived from petroleum oil and are made by scientists and engineers. Some examples are nylon, polyethylene, polyester, or Teflon. The main difference between artificial and natural polymers is that artificial ones can also take shapes that do not occur naturally, e.g. a branched polymer with a specific number of branches.
- The polymer field theory is a mathematical theory that can describe how polymers are arranged in space under various conditions. It has been demonstrated that computer simulations based on polymer field theories can produce beneficial outcomes, such as calculating the structures and properties of polymer solutions or thermoplastics.
Together with his colleagues Christos Likos and Roman Staňo, Smrek studies the structure, spatial arrangement, and dynamics of ring-shaped polymers by means of computational tools. "We examine these structures for mainly three reasons. Firstly, from a biological perspective: polymers can be found in nature, for example in plasmids. Secondly, we want to understand them theoretically", explains Smrek. Thirdly, the research team tries to shed light on the material perspective. "Because of the above-mentioned constraints, we found that materials made of ring polymers have some special mechanical properties and form interesting structures we want to find out more about", says the physicist. New insights into ring polymers are also interesting for the still young research field of DNA nanotechnology. Here, the sequence of DNA is used to form larger structures, such as the encapsulation of a drug. This way, the drug can reach its intended target in the body.
Finally, ring polymers seem promising for very soft and super stretchable materials that could be used for flexible electronics or soft robotics.
Nature’s most famous ring-shaped DNA
Bacteria and some other microscopic species contain plasmids, which are tiny circular DNA molecules. Physically distinct from chromosomal DNA, plasmids multiply on their own. They normally contain only a few genes, including some genes that are linked to antibiotic resistance. They can spread from one cell to another. Scientists use recombinant DNA techniques to splice the genes they want to study into a plasmid. The inserted gene is duplicated along with the plasmid as it duplicates itself.
Simulations pave the way for basic research
Jan Smrek and his team do basic research: Creating new material is not their primary goal. First and foremost they want to learn more about the physics of these special, circular structures. "I like to compare the intention of basic research with the one from cavemen who did not go out of their caves because they knew there would be antibiotics or iPhones, but simply because they were curious about what to find outside in our world", Smrek points out.
In a recently published paper, Jan Smrek and his co-authors examine how the ring shape of DNA molecules, their density, and their ability to carry charge affects their grouping into larger structures. To characterize the complex structures and properties of ring polymers the researchers run computer simulations using software they programmed themselves as well as software designed and maintained by other academics. "With our simulations, using supercomputers with many hundreds of processors, we can mimic the real-world physics in a computer. The parameters we have to set up are, e.g. the charge of the ions, the stiffness of the polymers, the temperature, and so on", Smrek explains.
Once the researchers have analyzed their results, the next step is to hand them over to their experimental colleagues, who will then design practical experiments with synthetic DNA to verify the theoretical predictions in the simulations.
Different charges lead to different structures
"In our simulations, we discovered that the DNA rings can form stacks. This is itself a bit counter-intuitive because the similar-charged rings should in principle repel and not fit nicely on top of each other. Furthermore, we found that the structure of the rings is dependent on the type of solvent in which they float when they are charged", says Smrek.
This means that the rings will self-assemble into a variety of forms depending on the charge. The charge itself can be generated through differently charged ions in the solvent. Density also influences the ring polymers. The density can be changed easily: To generate higher density, you put the polymers into a smaller container or equivalently compress the container to a smaller size.
If the charges are weak and the density is low, there are no stacks, and the rings float and form a liquid. In contrast, if the charge in the solution is very strong, it works like a glue between the rings, which then form longer and longer stacks, as the density is increased. As a result, the stacks can hardly move. They are stuck between each other. However, within the individual piles a single ring can still move and, for example, swap places with a ring from an adjacent stack. The researchers call this structure ‘cluster glass’. If the density is increased even further, the stacks even disappear.
In the broadest sense, these findings can help to better understand the physics of glass, one of the oldest man-made materials that is not yet fully understood.
Soap bubbles lead to the answer
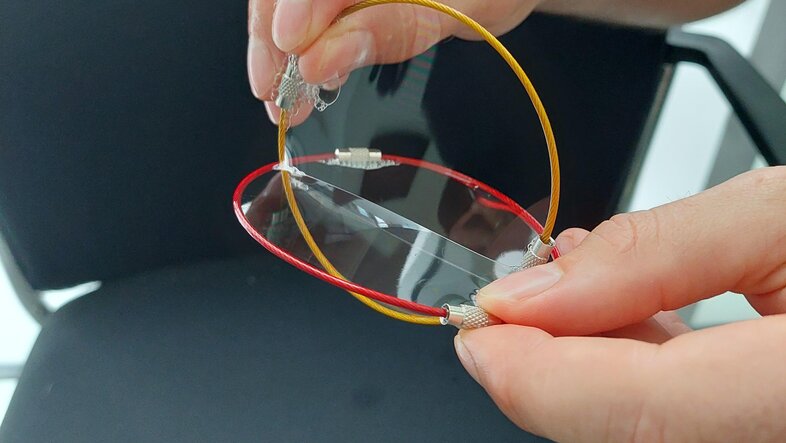
The so-called threadings are another intriguing characteristic the researchers were able to investigate with their simulations. These occur when one ring passes through another. "To recognize these structures within the simulations, you have to come up with a mathematical procedure, which is inspired by physics", says Smrek. To our surprise, he grabs a bowl of soap lye to demonstrate what he means. "We all played with soap bubbles – once you found the right concentration, you can easily make your own bubble liquid", he smiles. Then he bathes two metal rings in the soap water and shows how one ring pierces through the soap film of the other ring. "This is precisely what we do in our simulation. To observe the threadings, we computationally span a soap film on each ring. In this way, we can then find out if two rings interpenetrate", Smrek points out.
Surprises and difficulties lead to the next challenge
The research process can be very challenging and is often frustrating, but Jan Smrek has learnt how to deal with it. "As I also like to advice my students, 'Never complain'," Smrek laughs. "Of course, working as a scientist can be hard, but it is important to make a difference and tell the exciting side". For the researchers, it was very challenging to handle the huge amount of data they generated with their simulations. "You get a lot of results and you have to find the relevant outcomes in this huge amount of data. Roman Staňo, PhD student in our group and first author of the recently published paper, did a great job in finding sense in this huge mess", Smrek states.
Basic research comes with staying power – and now and then provides unexpected surprises. "We knew that the rings can form these stacks. Our expectations were: The denser the system, the longer these stacks will be. But we found that the stacks suddenly dissolve at low charge, if we increase the density – that was very surprising to us", Smrek reports. "A surprise is always exciting, because it tells us that we did not understand everything properly. It allows us to get a better understanding and leads us to our upcoming research goals".
Studying kinetoplasts will be the team's next task – a fascinating material made of very similar, short, charged DNA rings that can be linked together. In the future, the research group will investigate how the kinetoplast is organized in space and investigate its behaviour in different solutions.