Supercomputer, Schrödinger and the energy revolution
What do washing powder, car tyre rubber and paracetamol have in common? Like most of the materials we use in everyday life, they are not available as finished products from the start. They are based on source materials and produced by means of chemical reactions. For example, for the keyboard used to write this text, countless individual components (monomers) were linked to form polymers to create plastic.
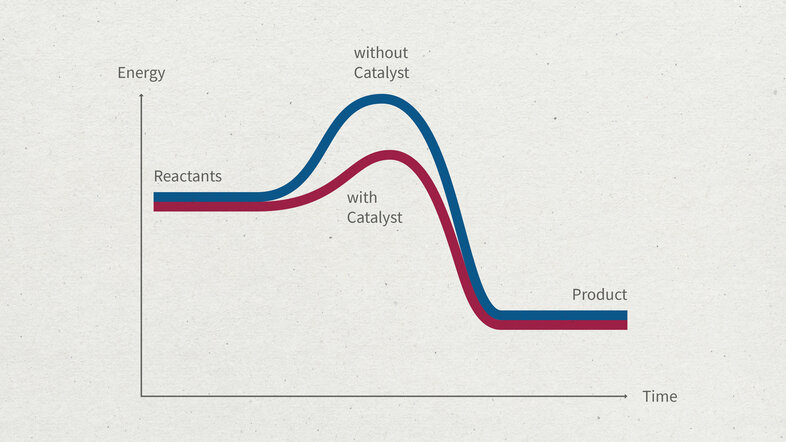
Chemical reactions need to overcome an energy barrier, the so-called activation energy. This is where a catalyst comes in. Catalysts are materials that facilitate a chemical reaction by lowering the activation energy (see illustration).
Computer simulations for a deeper understanding
Almost all chemical products thus rely on catalysis. Including those we may need for the energy revolution: One day we may be able to produce eco-friendly hydrogen at a large scale using solar energy. Today, the production of hydrogen still requires noble metals, such as platinum. But platinum is an expensive and rare raw material, which mainly comes from South Africa and Russia. "We simply do not have the amounts of platinum that we need to produce sufficient quantities of hydrogen," emphasises Georg Kresse, Professor of Computational Materials Physics at the University of Vienna.
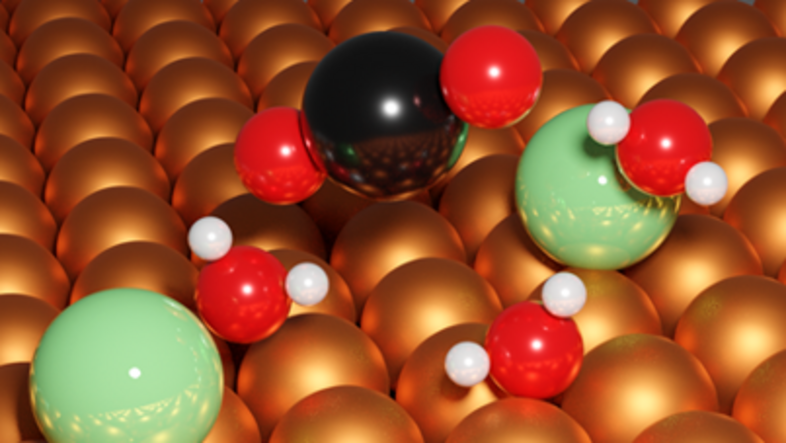
When searching for affordable and efficient alternatives, scientists can benefit from a profound understanding of the nano-scale events that occur as catalysis unfolds – step by step, atom for atom. At the point where we leave the realm of chemistry and enter the world of particle physics, Georg Kresse and his team come into play. "We simulate the individual steps of a catalysed reaction in the computer to try and understand catalysis from start to finish."
Optimising a catalyst usually requires time-consuming trial and error procedures. If it were possible to accurately predict the behaviour of materials using computer simulations, researchers could develop and improve suitable catalysts. This would entail enormous potential for innovation in research and industry. Be it green hydrogen, new chemicals or synthetic fuels made of carbon dioxide: More efficient catalysis could scale-up production in a resource-saving and economic way.
Close collaboration for the energy revolution
Understanding the processes that occur on the surface of materials is key, says Kresse. To produce hydrogen, water molecules are split in a process called electrolysis. This requires electricity, water and electrodes. The aim is to understand what happens at the interface between the electrode and the water as the reaction takes place. "We want to analyse the atomic processes in detail and use this knowledge to improve electrodes," explains Kresse.Ideally, these would be electrodes made of common, affordable materials instead of rare elements, such as platinum.
Collaboration is crucial at this interface between theory and practice. Kresse and his team can provide valuable theoretical input for researchers experimenting on catalytic materials. "By simulating in detail how a reaction unfolds on the catalyst, our colleagues can use this information to change parameters in a targeted way and optimise their experiments," says Kresse. The exchange of data between theory and practice often leads to a better understanding. Kresse and his team provide simulations and software solutions to other researchers and thus consider themselves enablers of practical applications.
Researching energy storage systems of the future
In the age of renewable energy, the question of energy storage is very central. Wind power and solar energy have a flaw: They are not available around the clock. Therefore, we need ways to store energy and make it available again in calm weather or at night. Batteries and pumped hydroelectric energy storage plants will not suffice to cover our requirements. The research groups involved in the Cluster of Excellence Materials for Energy Conversion and Storage focus on this challenge. They are developing innovative approaches to storing energy in chemical bonds: electrocatalysis and photocatalysis. Electrocatalysis uses green electricity to electrochemically convert water and CO2 to hydrogen, synthetic fuels and fine chemicals. Photocatalysis uses sunlight directly for chemical conversions – similar to plants during photosynthesis.
In the special research programme TACO (TAming COmplexity in Materials Modeling) at the University of Vienna and TU Vienna, theoretical and experimental scientists from the fields of chemistry, physics and process engineering are investigating fundamental processes that take place in complex materials.
The Schrödinger equation: no obstacle is unsurmountable
The starting point for Kresse's simulations is the probably most well-known formula of quantum mechanics: The Schrödinger equation. "For us, the Schrödinger equation is somewhat of a theory of everything. It describes everything we see and experience. Even the characteristics of almost all materials", explains Kresse.
Generally speaking, the equation describes the dynamics of a particle in a quantum system, for example an electron in a chemical bond. In theory, physicists can accurately predict the behaviour of any individual particle in a chemical reaction. However, there is a catch: When many particles are involved, as in any practical application, it is impossible to solve the equation accurately. Even with the fastest supercomputer.
For us, the Schrödinger equation is somewhat of a theory of everything. It describes everything we see and experience.Georg Kresse
But an insolvable equation is no obstacle for a theoretical physicist. Using special computation processes, Kresse and his team are able to approximate the solution of a Schrödinger equation. Their means of choice is the so-called density-functional theory (DFT), developed by Austrian-born Walter Kohn, who was awarded the Nobel Prize in chemistry for this achievement in 1998. "We combine the Schrödinger equation and the DFT, allowing us to efficiently predict processes involving up to 1,000 particles," explains Kresse. An important step.
Nevertheless, it is still necessary to find methods that approximate the solutions of the exact Schrödinger equation for a very large number of particles. “It is my dream to solve the Schrödinger equation for relevant materials and catalytic processes with sufficient accuracy. Maybe one day we will have a breakthrough using better methods than the DFT. But currently, the computing power required for it is still enormous.”
Machine learning as a game changer
The computer program tackling this computing effort is called VASP (Vienna ab initio simulation package). Kresse is the main author of the program, which is continuously being developed by his research group to provide it to other researchers. Studies suggest that VASP is the world’s most widely used program for quantum mechanical simulations of materials. It is run on the Vienna Scientific Cluster, Austria’s most powerful computer, which is able to run quadrillions of computing operations per second.
Despite state-of-the-art hardware, the computing power available to Kresse and his team is limited. However, recent developments in the field of machine learning give Kresse hope. “Machine learning triggered a revolution. Compared to a few years ago, we are now able to calculate individual steps in our simulations several orders of magnitude faster. It might soon be possible to truly understand a catalytic process. And thus, also to improve the materials that we use as catalysts or electrodes. I think we are closer to our goal than ever before.”