Stable, inexpensive and exotic: 2D crystals in a graphene sandwich
Rudolphina: Kimmo Mustonen, as an expert in nanostructured materials, you chose to give insights into a new material you and your colleagues have developed: 2D materials within a graphene sandwich. So, to begin with: What is a graphene sandwich?
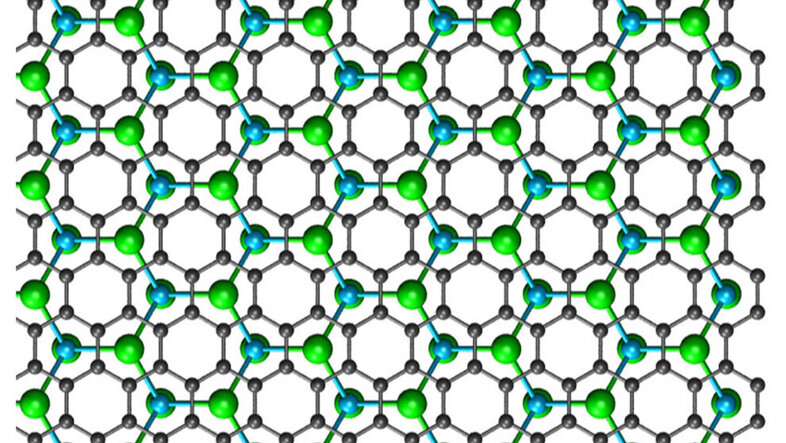
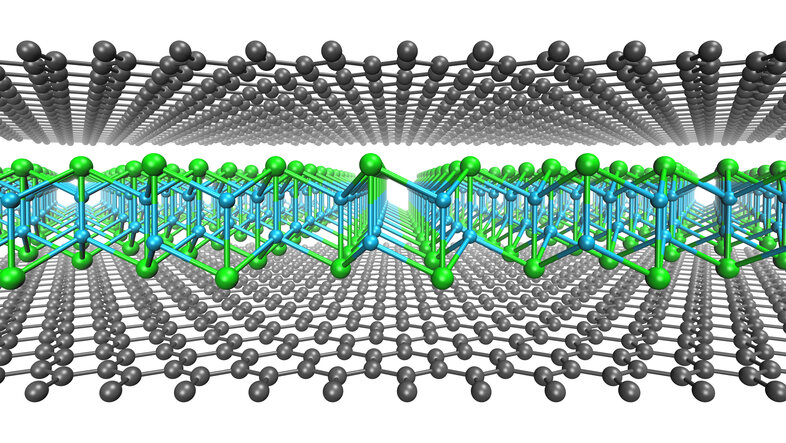
Kimmo Mustonen: 2D materials like graphene are atomically thin layers of atoms in a fully satisfied chemical state, meaning that they do not form covalent bonds with any other compounds. If you take two sheets of graphene and bring them together, they will stick due to the so-called 'van der Waals interaction' – that is the same force that allows the gecko lizard to walk on the ceiling –, but a tiny volume of space remains in between them. Using synthetic chemistry, it is possible to slightly increase this volume, and to grow other 2D compounds within it.
And that is exactly what we have done: Between two layers of graphene, we synthesised 2D crystals of copper and iodide, which would be completely unstable outside of the graphene and would immediately self-destruct. This method enables us to grow many other 2D materials of which some are immensely useful in applications, including in optics and catalysis. The graphene sandwich protects them from the surrounding chemical environment, making them very difficult to damage.
Rudolphina: Which three words best describe the new material?
Mustonen: Stable, inexpensive and exotic!
Rudolphina: Why should we know about the new 2D materials?
Mustonen: Inexpensive 2D materials that are suited for real-life applications other than exfoliated graphene are virtually non-existent. The growth technique that we utilise to make the graphene-encapsulated 2D copper iodide provides access to completely new materials that could not exist otherwise, and allows us to produce them in large quantities and at very low cost.
Rudolphina: What is the 'superpower' of the graphene sandwich?
Mustonen: The 'sandwiching' between the graphene layers protects the 2D materials synthesised inside from external agitations. For instance, when used to catalyse chemical reactions, the reaction byproducts will not poison the catalyst protected by a single layer of graphene. These materials are also completely air-stable, meaning that storing and manufacturing them is easy.
Rudolphina: What is still puzzling about this material?
Mustonen: The materials and the method we use to make them are completely new. We still do not quite know how complex and versatile 2D materials can be synthesised in graphene encapsulation. The chemistry is not yet well understood.
Rudolphina: What is the next step in your research?
Mustonen: We are currently working to understand the chemical processes taking place in the synthesis, and to find out the physical properties of the new materials to utilise them in suitable applications. These tasks necessitate the use of highly sophisticated and expensive research instruments, as they demand the capability to observe chemical processes and materials at the atomic and molecular scale.
Rudolphina: Why might the graphene-sandwiched 2D materials be important in our future?
Mustonen: Real-life devices, such as solar panels are large in size, and building them requires large amounts of material. The graphene-sandwiched 2D materials are the only class of currently existing 2D materials that satisfies the raw-material volume requirement and meets the cost point.
Rudolphina: What would you like to achieve with your research?
Mustonen: My own dream and vision is that, through energy-efficient and more environmentally friendly technologies enabled by 2D materials, our research could benefit both humankind and the planet on which we live.