Clean and reliable water: Looking to the sky for answers
Clean water is an indispensable yet underappreciated resource in the lives of many Europeans. Whether for washing your hands, brushing your teeth, cooking or brewing coffee: In countries where clean water is readily available, turning on a tap seems as mundane as flipping a light switch. Other regions of the world are not so lucky, yet a solution to the problem may be all around us.
The vast majority of water on Earth is undrinkable salt water (96.5 %), while most of the remaining freshwater is stored in glaciers and groundwater, which is difficult to access without significant energy use. In fact, only around 0.3 % of the Earth's total water supply is available as drinking water. As a result, vast areas of the planet suffer from water stress owing to over-usage, pollution and climate change.
Did you know...?
- According to the United Nations, more than 2 billion people gained access to safely managed drinking water since the year 2000.
- However, 26 % of the world's population still does not have reliable access to safely managed drinking water according to the UN World Water Development Report (2020 data).
- 25 countries housing a quarter of the world population face extremely high water stress each year (according to the Water Risk Atlas by the World Resources Institute)
- While it takes seconds to get water from a tap, UNICEF reported that 29% of the population in sub-Saharan Africa has to travel at least 30 minutes to access drinking water. The burden of fetching water falls disproportionately on women and girls.
- 22nd March 2024 will mark the World Water Day, a global awareness day held annually since 1993. The theme of World Water Day 2024 is ‘Water for Peace’. Clean water and sanitation for all is one of the 17 Sustainable Development Goals defined by the United Nations General Assembly in 2015.
Water, which many of us may consider a necessary yet abundant resource in our everyday lives, is a valuable and scarce commodity in many parts of the world, whose availability decides over life or death. The United Nations estimates that around a quarter of the world's population does not have reliable access to clean water, with forecasts predicting that the number of people affected may double over the next 25 years. To ensure reliable access to water in even some of the most extreme environments on Earth, researchers are taking on the task of developing innovative solutions to clean water production.
A "sponge" to draw water from the air
In my research group, we are looking to the sky for answers. More specifically, to air. Air is an abundant reservoir of water in its gaseous form, known as water vapour. The Earth's atmosphere at any given time contains around six times more water vapour than the combined volume of all the world's rivers. The challenge is harvesting this water from air when it is present in low concentrations, for example, in hot, arid environments susceptible to water stress.
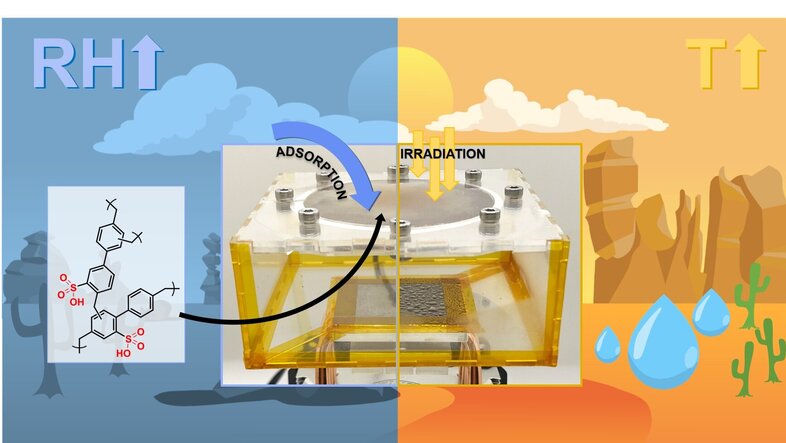
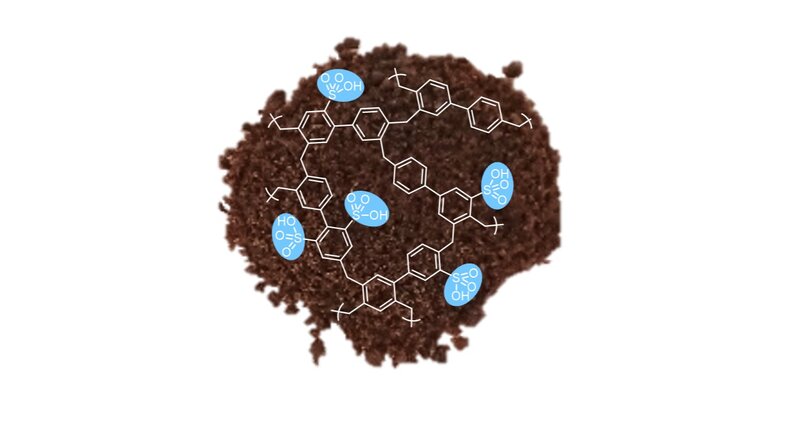
We have developed a highly porous polymer that proved much more efficient and cost-effective than previously known methods for "harvesting" water from the atmosphere. This highly cross-linked polymer, which we call SHCP-10, can adsorb water from air like a sponge.
Our "sponge" contains tiny pores, typically less than 20 nm in diameter, equivalent in size to the amount your fingernails grow roughly every 20 seconds. Packing many of these tiny pores into the polymer gives the material a huge surface area; 10 grams of SHCP-10 (enough to fit into a small coffee cup), has a surface area of around 7000 m2, which is equivalent to a full-size football pitch.
We envision a future where low-cost water harvesting devices are as common in water-stressed regions as air conditioning.Robert Woodward
In addition to its high surface area, the chemistry of SHCP-10 is highly hydrophilic, i.e. water loving. Combining these properties in a single material allows the polymer to pull water out of air and accumulate it at its surface. This process results in the adsorption and storage of up to 0.8 litres per kilogram of SHCP-10.
Combining these properties in a single material allows the polymer to pull water out of air and accumulate it at its surface. This process results in the adsorption and storage of up to 0.8 litres per kilogram of SHCP-10.
The network is so hydrophilic, in fact, that it can adsorb water even from the dry air present in extreme environments, making it an excellent candidate for use in water-stressed areas. In the laboratory, we could show that SHCP-10 adsorbs around 0.3 litres of water per kilogram of polymer even under Saharan conditions, i.e. with less than 40 % humidity and temperatures over 40 ºC.
Quick, reliable and energy-saving
In addition to the passive adsorption of large amounts of water, one of the critical advantages of SHCP-10 is that the adsorbed water is bound to the polymer’s surface via weak physical interactions rather than strong chemical bonds. Overcoming these weak physical interactions to desorb and recover the water requires significantly less energy compared to conventional methods that condense water directly from air by cooling (see box below). Simply exposing water-loaded SHCP-10 to sunlight is enough to trigger the desorption of the clean, pure water for collection.
Explained: Water condensation by cooling
There are many conventional methods of extracting water vapour from the air. One is water condensation - turning water vapour into liquid: this is achieved by cooling the air below a certain temperature, known as the dew point. The dew point depends on the humidity of the air - the lower the humidity, the lower the dew point. This means that dry desert air must be significantly cooled in order to extract water from it. Depending on the cooling device used, this can be energy intensive.
The relatively fast adsorption-desorption process (~2 hours total) allows for several uptake-discharge processes per day, drastically improving daily water harvest yields. Furthermore, SHCP-10 displays excellent stability and does not degrade even after hundreds of adsorption-desorption cycles.
All these factors make our material a significant milestone in the further development of water extraction from air. Adsorbents for atmospheric water harvesting, such as SHCP-10, represent an alternative water source which, in addition to being location-independent, produce water that requires significantly less purification than groundwater, if any at all.
Our work is now focussing on the design of more sophisticated devices utilising SHCP-10 for harvesting water from air at a larger scale, aiming to impart the performance of the polymer in an idealised laboratory environment to a user-friendly product. We envision a future where low-energy water harvesting devices capable of supplying multiple litres of water per day may be as familiar a sight in water-stressed regions as air-conditioning units.
After establishing his independent research profile in the Department of Chemical Engineering at Imperial College London, he joined the University of Vienna in 2020, where he continues to develop his research profile.