Nanomagnets to fight cancer
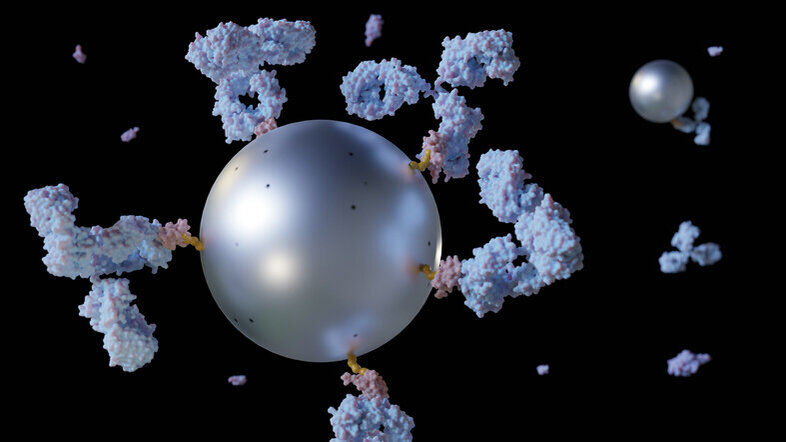
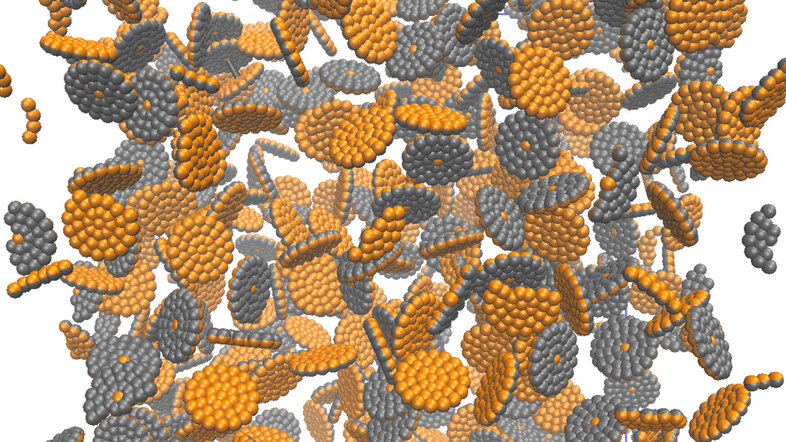
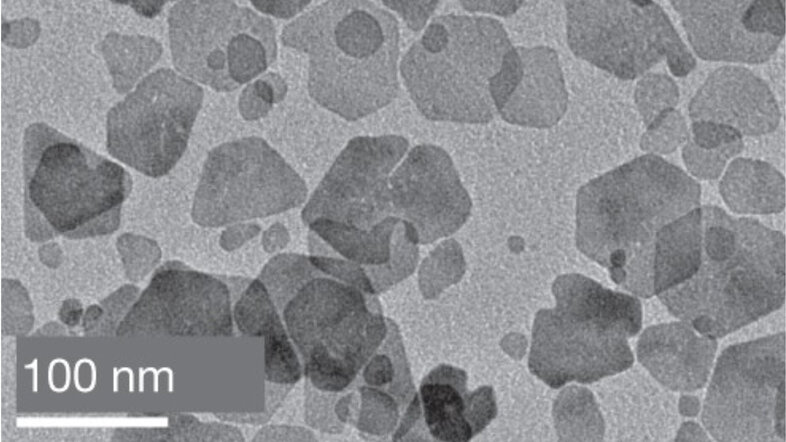
Size and shape matter: Whether it is a child trying to fit the right block through the appropriate opening of a shape sorting game, or a researcher working with nanoscale materials, such as magnetic nanoparticles. With sizes ranging from 10 to 100 nanometres, these particles are at least ten million times smaller than a meter. Just as a child finds the right shape, researchers combine repeated experiments and careful analyses to determine the optimal shapes and sizes of the magnetic nanoparticles to transport them through blood vessels and into living cells.
Biomedical Applications
One of the main ideas behind magnetic nanoparticle research is that when dispersed in a liquid, they can be moved around with the help of an applied magnetic field. This property might come in handy in cancer medicine if the particles are coated with medications and injected into the bloodstream. We can then transport magnetic nanoparticles to a damaged organ or tumour and hold them there with an external magnetic field until the drug takes effect. This technique is called drug targeting or targeted drug delivery.
Alternatively, after being delivered to the target, controlled rotation of nanoparticles that follow an additionally applied oscillating magnetic field can cause local heating (hyperthermia) that might help shrink the tumour. This is how magnetic hyperthermia can be used to treat cancer.
There are two main benefits of these methods compared to standard chemo- or radiotherapies. First, the effect of the medication is highly localised and does not destroy healthy tissues; second, magnetic nanoparticles can facilitate the immediate tomographic visualisation of tumours.
Getting nanoparticles to their destination
As simple as these solutions may seem, the biomedical application of magnetic nanoparticles raises numerous theoretical and practical questions. Researchers grapple with determining the precise size of particles needed, as well as the optimal structure and shape to reach the target or generate heat. They also need to consider the risk of particle clustering and subsequent blood vessel blockage, and whether controlled clustering could make the particles more responsive to applied magnetic fields.
Practical issues include ensuring non-toxicity, devising methods for cellular penetration, and developing strategies for removing the particles after completing their mission, such as degradation into harmless or beneficial compounds.
How computational modelling comes into play
It is crucial to address these questions if we want to establish magnetic nanoparticle-based systems as materials of the future. As in the shape sorting game, achieving this requires a blend of repeated experiments and meticulous analyses. In practice, modern research involves collaboration across disciplines, bringing together experts in soft matter, bio-, condensed, and soft-matter physics, chemistry and mathematics.
This collaboration merges theoretical and experimental efforts with clinical trials. In this context, my research group specialises in computational and analytical modelling of magnetic nanoparticle-based systems, aiming to understand how the shape and internal structure influence their behaviour. This research not only streamlines experimental trials and optimises material synthesis, but also provides a fundamental understanding of nanoparticle magnetism.
From models to hospitals
The behaviour of magnetic nanoparticles in liquids has been studied for almost 60 years. For over 25 years, researchers have worked towards enabling clinical trials.
However, since 2020, the pandemic and wars have shifted priorities, sparking debates on funding for fundamental research and raising ethical concerns about animal trials, as well as the sustainability of magnetic nanostructured materials. Furthermore, artificial intelligence (AI) has significantly permeated our daily lives, posing a challenge to traditional research methods.
Careful integration of AI technology
As we learn to handle the new reality, I advocate careful use of the knowledge we have accumulated, while integrating new methods, such as AI. Rather than blindly replacing traditional approaches, AI should complement and optimise them, as suggested in our recently proposed research initiative (Micro)Magnetism and (Quantum)Materials (MAGAMAT).
MAGAMAT aims to unite researchers across Austria and Germany to put forward new strategies for developing sustainable magnetic materials with desired properties. For this initiative in particular, and for all scientific progress in general, it is vital to maintain international collaboration and to promote unity among researchers worldwide, irrespective of gender, nationality or beliefs.