Environmental protection within our body
Microbes - aren't they the invisible pathogens that live in the dirt? "In the early 2000s, microbiologists began using new technologies to analyse the microbes that live in, on and all around us – and were surprised by the huge number and diversity of organisms they found," explains David Berry, who studies the gut microbiome at the Department of Microbiology and Ecosystem Science.
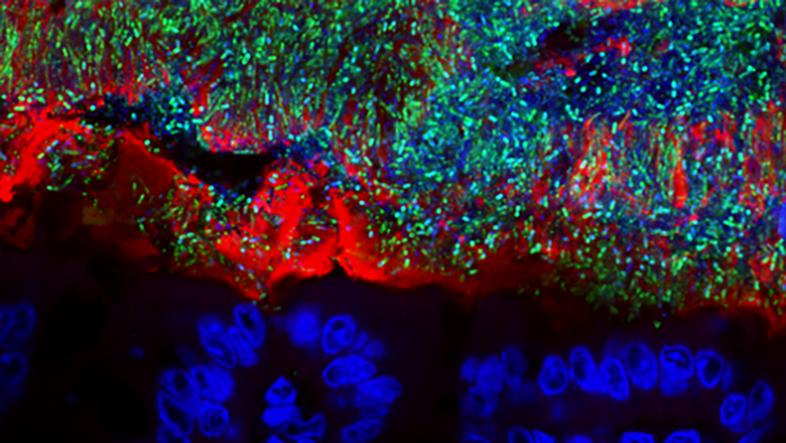
For Berry, two key facts about microbes – a collective term for a wide variety of microscopically small life forms from the domains of bacteria, archaea and eukaryotes – stand out: First, they are many and they are everywhere. There are more microbial cells in and on the human body than human cells. There is hardly a surface that is not covered by them. And: "Most microbes are completely harmless to useful. Pathogens, i.e., disease-causing agents, are strongly in the minority."
The rehabilitation of the former villains
Bacteria in particular used to be considered the 'bad guys'. With increasing research into the microbiome since the early 2000s, it has been recognised that many bacteria are actually necessary for the healthy development and functioning of the body.
"We are born sterile, but in the first two weeks we pick up microbes, through birth as well as from the environment, from other people, animals, from surfaces. Our microbiome continues to be influenced by breast milk, baby milk and by solid food later on," Berry explains. In this way, each person develops an individual 'microbial fingerprint' in the course of the first two years of life.
The gut-microbiome-brain axis
Berry uses the example of extreme pre-term births to illustrate the importance of the developing microbiome, "The composition of the microbiome one month after a preterm birth is predictive for cognitive and social functions two years later." In adults, too, a change in the microbiome can be observed in neurodegenerative diseases and even in depression.
In animal models, this connection could be studied more clearly. There are strains of mice that are characterised by their shy behaviour and those that are very curious. If you exchange their gut microbiome, the shy mice will become curious and the curious ones shy, Berry describes his 'favourite experiment'. "The microbial phenotype has an influence on behaviour," Berry is fascinated.
In microbiome research, this connection is called the 'gut-microbiome-brain axis' and research into the mechanisms behind it is currently a hot topic in the field. Microbiologists like Berry consider the immune system a likely mediator.
After a PhD in Environmental Engineering at the University of Michigan, he moved to the Department of Microbiology and Ecosystem Science at the University of Vienna in 2009. He is a member of the scientific advisory board of the Austrian Microbiome Initiative AMICI and on the board of directors of the Joint Microbiome Facility (JMF) of the University of Vienna and the Medical University of Vienna.
From battlefield to garden
As the view of bacteria has changed, so has the way microbiologists view the immune system. Berry explains, "What was previously seen as a sentry or killing system of intruders, we now see as a gardener, keeping a balance between the function of the microbes and the body."
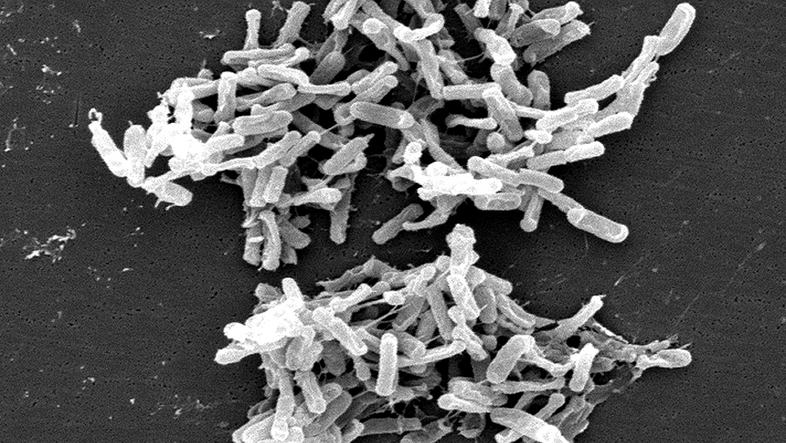
And finally, the concept of 'pathogens' (disease-causing agents) has also undergone a transformation in this context, the microbiologist says. Where a wide variety of microbes have a place in our body's garden, pathogens are often simply opportunists that become dangerous under certain circumstances (of the immune system) but are harmless in other situations, he says. Berry mentions Clostridioides difficile as an example. This so-called 'hospital germ' is the cause of an infection called CDI, a recurring and serious diarrhoeal disease. "However, many babies in particular have the bacterium in their intestines and do not show any illness," says the microbiologist.
The important thing is how CDI occurs: Most patients contract CDI after being treated with broad-spectrum antibiotics. "Broad-spectrum antibiotics destroy not only 'pathogens', but also the beneficial microbiome, which also plays a protective role. Because in an 'empty house', C. diff. can spread much better than in an already well-populated one," says Berry.
A healthy microbiome in a healthy body
Thus, the microbiome is central to the health of our body – ranging from the protection against the spread of individual bacteria to our brain function. Therefore, microbiologists developed the idea of treating patients having a dysfunctional microbiome by transplanting a healthy microbiome. This treatment, known as 'fecal microbiota transplant’, was approved by the US Food and Drug Administration (FDA) in 2018 and has been used successfully under very strict conditions since then, including meticulous screening and cleaning precautions and rare side effects.
However, Berry warns against an unqualified generalisation of the idea that people should increase their exposure to the microbiome of others, "As late as the 19th century, there were still rampant typhoid and cholera epidemics in the US and Europe, caused largely by the exchange of faeces via contaminants, especially in the water. In the early 20th century, drinking water was chlorinated and with that, these incidents also dropped tremendously. We should keep this in mind when people say that our modern way of life is 'over-sterilised' and romanticise a more 'original' lifestyle."
But all this raises a new question: What actually is a healthy microbiome? Because: "Against the backdrop of the individual interactions between the body and the microbiome, it can be possible that a certain microbial composition works well in one person, but leads to illness in another," Berry explains the problem.
This, however, is above all an opportunity for personalised medicine. Berry says, "If we better understood the interactions between the body and the microbiome, we could tell which microbes are helpful or harmful in a given situation by looking at a patient's genetic profile."
The enemy of my enemies is my friend
But when it comes to controlling the gut microbiome in a particular way, new tools are needed. As we already know, antibiotics – as necessary as they sometimes are – also have the potential to cause great damage to the microbiome through their undifferentiated effect.
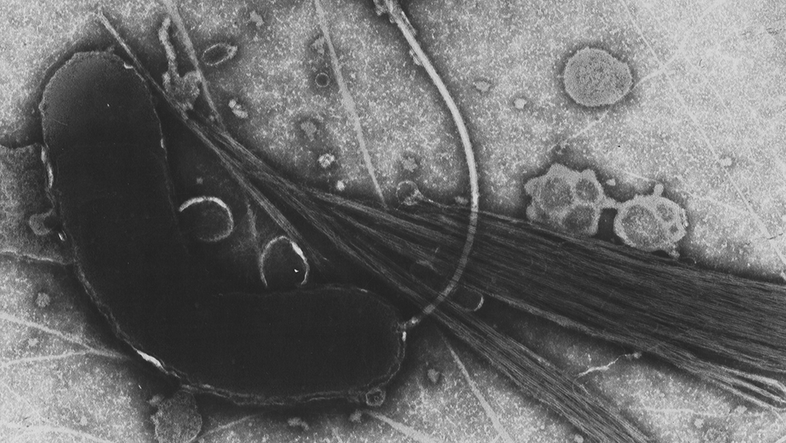
Berry sees the so-called 'phage therapy' as a promising new approach, "Phages are viruses that infect bacteria. They are so specific that they can attack individual bacterial strains, giving us the opportunity to really fine-tune the microbiome." Berry sees the potential of phage therapy not only where antibiotics are causing too much damage, but also where antibiotics can no longer do any good, i.e. in case of multi-resistant pathogenic bacteria.
Keeping an eye on the big picture with green and red microbiology
With multi-resistant pathogens, the circle is closed with the environment that is in us and the one that surrounds us. These 'super bugs' develop when bacteria that are exposed to antibiotics enter nature through our waste water and, above all, livestock farming.
Berry traces the causal chain, "The bacteria that are already potential human pathogens now come into contact with bacterial communities in the soil that have resistance genes for these antibiotics. And because of the strong selective pressure exerted by the huge quantities of antibiotics, it is advantageous for the potential pathogens to acquire the resistances and also pass them on – until they eventually reach humans again."
And this also brings together two areas of research that have traditionally been studied separately: 'red' microbiology, which deals with medically relevant mechanisms and methods, and 'green' microbiology, which focusses on the environment. "This is also the basic idea in our new Cluster of Excellence 'Microbiomes Drive Planetary Health'," explains the microbiologist. "We want to look at the microbiome more holistically and integrate the mechanisms of action of the individual aspects as well as the methods of the different disciplines." No matter whether it is inside us or around us, the health of the microbial environment is essential for human health. (ds)